20+ Enthalpy Bond Energy Table US
20+ Enthalpy Bond Energy Table US. The bond dissociation energy (enthalpy) is also referred to as bond disruption energy, bond energy, bond strength, or binding energy (abbreviation: The exact bond enthalpy of a particular chemical bond depends upon the molecular environment in which the bond exists.
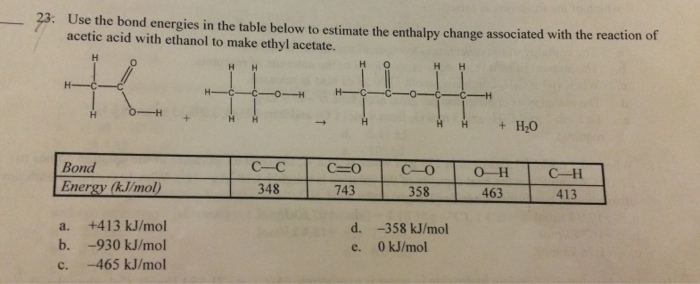
To calculate bond energy for molecules in a liquid state, you need to also look up the enthalpy change of vaporization for the liquid molecule.
Conversely, energy is released when a bond forms between two gaseous atoms or molecular fragments. Conversely, energy is released when a bond forms between two gaseous atoms or molecular fragments. The diagram above shows, in terms of a dot and cross diagram, the breaking of a. The bond dissociation energy (enthalpy) is also referred to as bond disruption energy, bond energy, bond strength, or binding energy (abbreviation:
Posting Komentar untuk "20+ Enthalpy Bond Energy Table US"